Topics
- pH
- Dilution
- Acids
- Bases
Description
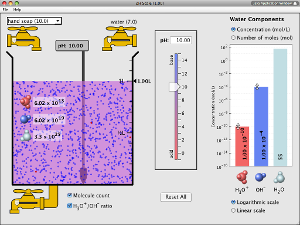
Test the pH of things like coffee, spit, and soap to determine whether each is acidic, basic, or neutral. Visualize the relative number of hydroxide ions and hydronium ions in solution. Switch between logarithmic and linear scales. Investigate whether changing the volume or diluting with water affects the pH. Or you can design your own liquid!
Sample Learning Goals
- Determine if a liquid is acidic, basic, or neutral
- Place acids or bases in relative order
- Describe on a molecular scale, with illustrations, how the water equilibrium varies with pH
- Determine concentration of hydroxide, hydronium and water at a given pH
- Relate liquid color to pH
- Predict (qualitatively and quantitatively) how dilution and volume will affect the pH and concentration of hydroxide, hydronium and water
Version 1.04